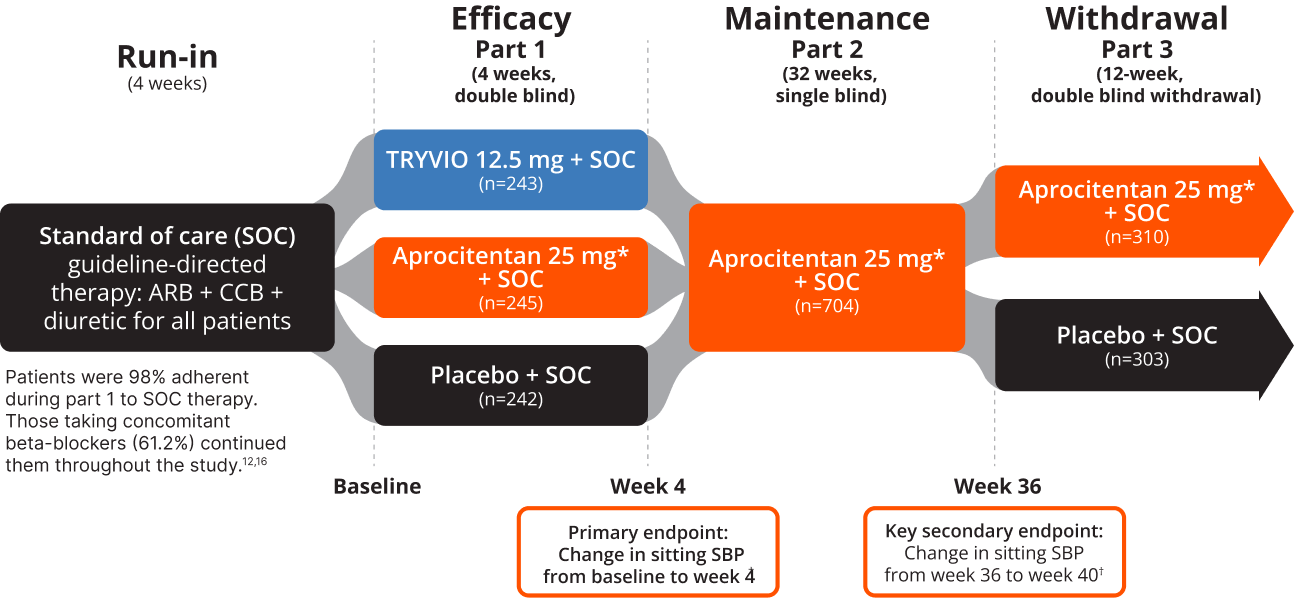
PRECISION: A robust study in patients with difficult-to-control HTN despite receiving >= 3 guideline-directed background BP therapies1-3.
KEY INCLUSION CRITERIA
- Unattended SiSBP ≥140 mm Hg
- History of difficult-to-control BP despite ≥3 antihypertensive medications, including a diuretic, for at least 1 year
KEY INCLUSION CRITERIA
- Confirmed hypertensive crisis (BP >180/120 mm Hg)*
- Major cardiovascular, renal, cerebrovascular medical complications in the past 6 months or NYHA stage III-IV heart failure
- N-terminal pro-BNP levels ≥500 pg/mL
- eGFR < 15 mL/min/1.73 m2
Confirmed hypertensive crisis (grade 3) defined as SiSBP ≥180 mm Hg and/or sitting diastolic blood pressure (SiDBP) ≥110 mm Hg as measured by AOBPM at 2 different timepoints.
AOBPM = automated office blood pressure measurement; BP = blood pressure; eGFR = estimated glomerular filtration rate; NYHA = New York Heart Association; pro-BNP = pro-B-type natriuretic peptide; SBP = systolic blood pressure; SiSBP = sitting office systolic blood pressure.
PRECISION subjects were a diverse, high-risk population1,3
AHT = antihypertensive; eGFR = estimated glomerular filtration rate; UACR = urine albumin-to-creatinine ratio.
References: 1. TRYVIO® (aprocitentan) [prescribing information]. Radnor, PA: Idorsia Pharmaceuticals US Inc; 2025. 2. Danaietash P, Verweij P, Wang H, et al. Identifying and treating resistant hypertension in PRECISION: A randomized long-term clinical trial with aprocitentan. J Clin Hypertens (Greenwich). 2022;24(7):804-813. doi:10.1111/jch.14517 3. Schlaich MP, Bellet M, Weber MA, et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022;400(10367):1927-1937. Published correction appears in Lancet. 2023;401(10373):268. doi:10.1016/S0140-6736(22)02034-7 4. Data on File. PRECISION CSR. Idorsia. 2022.
IMPORTANT SAFETY INFORMATION & INDICATION

- TRYVIO is contraindicated for use during pregnancy because it may cause fetal harm if used by pregnant patients. Therefore in patients who can become pregnant, exclude pregnancy prior to initiation of TRYVIO.
- Advise use of effective contraception before the start of TRYVIO, during treatment and for one month after stopping treatment.
- When pregnancy is detected, discontinue TRYVIO as soon as possible.
CONTRAINDICATIONS
TRYVIO is contraindicated:
- in patients who are pregnant.
- in patients who are hypersensitive to aprocitentan or any of its excipients.
WARNINGS AND PRECAUTIONS
Embryo-Fatal Toxicity
Based on data from animal reproduction studies with endothelin receptor antagonists (ERAs), TRYVIO may cause fetal harm when administered during pregnancy and is contraindicated for use in patients who are pregnant. The available human data for endothelin receptor antagonists do not establish the presence or absence of fetal harm related to the use of TRYVIO. Counsel patients who can become pregnant about the potential risk to a fetus. Obtain a pregnancy test prior to initiation of treatment with TRYVIO. Advise patients who can become pregnant to use effective contraception during treatment, and for one month after the final dose of TRYVIO. When pregnancy is detected, discontinue TRYVIO as soon as possible.
Hepatotoxicity
Elevations of aminotransferases and hepatotoxicity are known effects of ERAs, including TRYVIO. Elevations in alanine transaminase (ALT) or aspartate aminotransferase (AST) of greater than 5-fold upper limit of normal (ULN) were observed rarely in patients treated with aprocitentan in the clinical trial, including cases with positive rechallenge. To reduce the risk of potential serious hepatotoxicity, measure serum aminotransferase levels and total bilirubin prior to initiation of treatment and repeat during treatment periodically and as clinically indicated.
Do not initiate TRYVIO in patients with elevated aminotransferases (>3 × ULN) or moderate to severe hepatic impairment. Advise patients with symptoms suggesting hepatotoxicity (nausea, vomiting, right upper quadrant pain, fatigue, anorexia, scleral icterus, jaundice, dark urine, fever, or itching) to immediately stop treatment with TRYVIO and seek medical attention.
If sustained, unexplained, clinically relevant aminotransferase elevations occur, or if elevations are accompanied by an increase in bilirubin >2 × ULN, or if clinical symptoms of hepatotoxicity occur, discontinue TRYVIO.
Fluid Retention
Fluid retention and peripheral edema are known effects of ERAs, including TRYVIO. Edema/fluid retention was reported in 9% of TRYVIO-treated patients compared with 18% of patients receiving aprocitentan 25 mg (twice the recommended dose) and 2% on placebo in the clinical trial, requiring additional diuretic use in some patients. Older age and chronic kidney disease are risk factors for edema/fluid retention with TRYVIO. TRYVIO has not been studied in patients with heart failure New York Heart Association stage III–IV, unstable cardiac function, or with NTproBNP ≥500 pg/mL. TRYVIO is not recommended in these patients.
Monitor for signs and symptoms of fluid retention, weight gain, and worsening heart failure. If clinically significant fluid retention develops, treat appropriately, and consider discontinuation of TRYVIO.
Hemoglobin Decrease
Decreases in hemoglobin concentration and hematocrit have occurred following administration of other ERAs and were observed in the clinical trial with TRYVIO. Hemoglobin decreases usually presented early, stabilized thereafter, and were reversible after discontinuation. A decrease in hemoglobin of >2 g/dL from baseline was observed in 7% of patients compared to 1% of placebo patients. A decrease to below 10.0 g/dL was observed in 3% of TRYVIO-treated patients compared to 0 patients taking placebo. Initiation of TRYVIO is not recommended in patients with severe anemia. Measure hemoglobin prior to initiation of treatment and periodically during treatment as clinically indicated.
Decreased Sperm Counts
TRYVIO, like other ERAs, may have an adverse effect on spermatogenesis. Counsel men about potential effects on fertility.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions (more frequent than placebo and ≥ 2% in TRYVIO-treated patients) are edema/fluid retention and anemia.
USE IN SPECIFIC POPULATIONS
Lactation
There are no data on the presence of aprocitentan in human milk, the effects on the breastfed infant, or the effect on milk production. Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment with TRYVIO.
Renal Impairment
TRYVIO is not recommended in patients with kidney failure (eGFR <15 mL/min) or on dialysis. Patients with renal impairment are at increased risk of edema/fluid retention.
Hepatic Impairment
TRYVIO is not recommended in patients with moderate and severe hepatic impairment (Child-Pugh class B and C) because these patients may be at increased risk for poor outcomes from hepatotoxicity.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. Please see full Prescribing Information including BOXED Warning and Medication Guide.
INDICATION
TRYVIO is an endothelin receptor antagonist indicated for the treatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugs. Lowering blood pressure reduces the risk of fatal and non fatal cardiovascular events, primarily strokes and myocardial infarctions.